Inspired Drug Delivery
Nova Thin Film Pharmaceuticals is realizing the potential
of thin film technology
of thin film technology
About us
Nova Thin Film Pharmaceuticals is a specialty drug delivery company that develops and manufactures dissolving thin films using patented DepoFilm™ technology.
NOVA Thin FIlm Pharmaceuticals (NTFP) is on a mission to solve drug delivery challenges to address unmet clinical needs by unlocking the economic and technical potential of transmucosal soluble films. Founded by management who pioneered the thin film drug delivery industry, NTFP employs patented DepoFilm™ technology to fulfill its mission.
DepoFilm solves the challenges of conventional solution casting manufacturing, and enables novel film compositions. NTFP is advancing a robust pipleine of thin film products and working with a select group of partners on co-development projects. NTFP is based in North Carolina, with formulation laboratories in Greensboro and manufacturing in Wilmington.
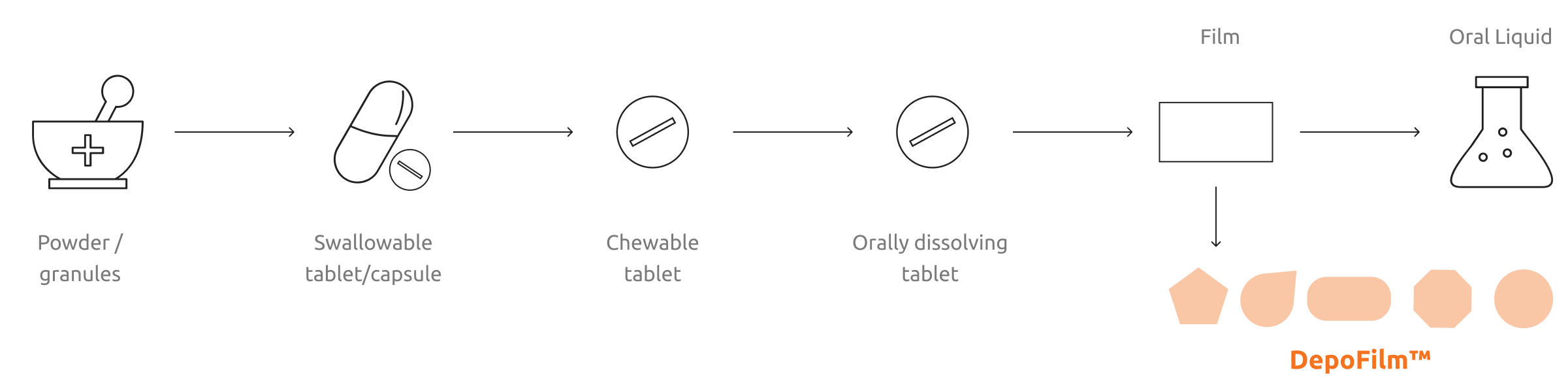
Film












Swallowable tablet/capsule
DepoFilm™
Chewable tablet
Orally dissolving tablet
Powder /
granules
granules
Oral Liquid
Our Team
Our management team and board is composed of a group that essentially created the modern oral soluble film industry.
Management
Board of Directors
Scientific Advisors
Joseph Fuisz
JD
Mr. Fuisz serves as our Chief Executive Officer. Mr. Fuisz has worked in the pharmaceutical industry for eighteen years. He named inventor on over forty-five issues US patents, including the largest patent estate related to wet-cast films. Mr. Fuisz co-founded the world’s largest wet-cast thin film company and served as its Senior Vice President for Business Development. Subsequently, Mr. Fuisz co-founded a company acquired by a leading global consumer products company based on Fuisz-patented extruded sheet technology. Mr. Fuisz also served as CEO of a vaginal drug delivery company acquired by a global pharmaceutical company. Joseph Fuisz has a BA from Yale University and a JD from the Columbia University School of Law. He is a member of the bar in the State of New York and the District of Columbia, and a Slovene American.
Madhu Hariharan
PhD
Dr. Hariharan serves as both our Chief Operating Officer and Chief Scientific Officer. He is a pharmaceutical industry executive with diverse work experience in product development, regulatory filing, technical operations and commercial launch of Rx, generic and OTC pharmaceuticals and nutraceuticals. He has worked in the area of complex drug delivery systems for the majority of his 25 years in the pharmaceutical industry, serving in a variety of technical leadership positions at specialty pharma companies as well as ethical and generic pharma companies. His expertise is in the area of pre-formulation, formulation, technical operations, regulatory filings and strategy for soluble films, implants and other complex dosage forms. He is the author of several scientific publications and is an inventor on a number of patents in the area of drug delivery. Madhu Hariharan has a Bachelor’s degree in Pharmacy and a Ph.D. in Pharmaceutics.
Kurt Lauenstein
MD
Kurt Lauenstein is our Chief Medical Officer. He is a graduate of Harvard College and the Vermont Medical School and completed his residency at Cone Hospital in Greensboro. Dr. Lauenstein is an expert in the area of neurotransmitters and receptors in the brain and the intersection of statistical analysis and clinical practice. As a clinician, Dr. Lauenstein experience on the front lines of the opiate epidemic in his clinical practice has made him a keen proponent of mental health and community needs for emotional well-being as an adjunct to pharmacological intervention. Dr. Lauenstein also serves on the oversight committee of the Better Evidence Project, based at George Mason University, which is developing a library cataloguing peacebuilding efforts of organizations around the world.
Yousry Sayed
PhD
Dr. Sayed was a longtime faculty member in the chemistry and biochemistry department, a student mentor and the former director of the General College, now known as University College of the University of North Carolina, Wilmington. Dr. Yousry Sayed is the founder and CEO of Quality Chemical Laboratories (QCL), one of the leading pharmaceutical CDMOs in the US. QCL is a partner and lead investor in NTFP.
Richard C. Fuisz
MD
Richard Fuisz M.D. has been a principal architect of two novel dosage forms: orally disintegrating tablets, and orally soluble films. He founded and served as CEO of two public healthcare companies, both acquired. He is an inventor on over 130+ issued United States patents, principally relating to drug delivery but also to diverse fields including electronic mail, computer databases, cipher systems, and others. Dr. Fuisz’s inventions include the largest patent estate relating to wet-cast films. Dr. Fuisz is a graduate of Georgetown College and Georgetown Medical School, and a Slovene American.
Giovanni Pauletti
PhD
Dr Pauletti is currently the Gustavus and Henry Pfeiffer Endowed Professor and Chair of the Department of Pharmaceutical and Administrative Sciences at the St. Louis College of Pharmacy. Dr Pauletti has dedicated his academic research career to improve safety and therapeutic efficacy of drug interventions in patients by applying innovative drug delivery technologies.
Thierry Bilbault
PhD
Dr. Bilbault has 35+ years of experience in pharmaceutical development and technical operations including manufacturing and global supply chain. He has held numerous senior level CMC positions at Novartis, Pfizer, Galderma, Cynapsus and Sunovion. He is a recognized expert in the area of soluble thin film having been involved in the development of numerous consumer and prescription oral and sublingual film products at Pfizer, Novartis and Cynapsus /Sunovion. He advises NTFP on technical development matters as well as corporate and regulatory strategy. Dr. Bilbault is a native of France and resides in Florida.
Andrew Finn
Pharm.D
Dr Finn has 35 years of experience in pharmaceutical and clinical development experience. He has served in a variety of senior scientific roles at Glaxo, Solvay, Pozen, BDSI and AviorBio. He has numerous publications and patents in the area of clinical research and drug delivery.
Mandar Kodgule
PhD
Dr Kodgule is a pharmacist and medicinal chemist by training. He has over 25 years of experience with leading pharmaceutical companies such as Wockhardt, Glenmark, Ranbaxy in leadership roles in the areas of Chemical research, Formulation development, Intellectual Property, Corporate Strategy, Alliance Management, Merger & Acquisition, Business Development and Budget planning. He also founded IQGenX, a pharmaceutical CRO in Mumbai, India. Dr. Kodgule advises NTFP on API matters, generics development strategy as well as IP and litigation matters.
Phyllis Gardner
M.D.
Dr. Gardner has spent more than 35 years in academia, medicine and industry. She has served on the board of directors of several public and private companies, including Revance Therapeutics, Inc. since 2006, Corium International, Inc. from November 2007 to December 2018, and CohBar, Inc. from February 2019 to present. Dr. Gardner has also served as an advisor to Change Health Care, Inc. from April 2019 to present. From June 1999 to July 2014, she served in various capacities including as an Adjunct Partner at Essex Woodlands Health Ventures, a growth equity firm that focuses on the healthcare industry (and a predecessor firm to EW Healthcare Partners, a holder of MiMedx Series B Preferred Stock). Additionally, Dr. Gardner has been a member of the Harvard Medical School Board of Fellows since April 2013 and is a scientific reviewer for the Cancer Prevention and Research Institute of Texas.
She began her academic medical career at Stanford University, where she has held several positions including Senior Associate Dean for Education and Student Affairs and remains today as Professor of Medicine. From 1994 to 1998, she took a leave of absence from Stanford University to serve as Principal Scientist, Vice President of Research and Head of ALZA Technology Institute, a major drug delivery company. Dr. Gardner earned a Bachelor of Science degree in Biology from the University of Illinois and a Doctor of Medicine degree from Harvard Medical School. She trained in Internal Medicine at Massachusetts General Hospital, followed by Chief Residency at Stanford University Hospital and post-doctoral fellowships at Columbia University and University College London.
She began her academic medical career at Stanford University, where she has held several positions including Senior Associate Dean for Education and Student Affairs and remains today as Professor of Medicine. From 1994 to 1998, she took a leave of absence from Stanford University to serve as Principal Scientist, Vice President of Research and Head of ALZA Technology Institute, a major drug delivery company. Dr. Gardner earned a Bachelor of Science degree in Biology from the University of Illinois and a Doctor of Medicine degree from Harvard Medical School. She trained in Internal Medicine at Massachusetts General Hospital, followed by Chief Residency at Stanford University Hospital and post-doctoral fellowships at Columbia University and University College London.
Rick D’Augustine
MBA
Rick has over three decades of medical-industry experience in general management, venture capital investing and technology and business development in both public and private companies. He has served in a variety of C-Level capacities, including President & CEO, Chairman and Director of several private life-science companies. Previously, he spent five years as a group director at Senmed Medical Ventures, a private medical venture capital and business development firm. D’Augustine spent 16 years with Johnson & Johnson and was a founding board member of Cincinnati-based Ethicon Endo-Surgery, initially serving as vice president of administration and CFO, and subsequently as vice president of business development. He was a founding board member of Enable Medical Corp., a private, high-technology medical device company, as well as Atricure Inc., an Enable spinoff focused on surgical treatment of atrial fibrillation, which is now a public company. Currently, Rick is an independent consultant focused on early stage companies and technologies, and serves as a director for Enable Injections, Inc. and Ischemia Care Diagnostics, LLC. He has a bachelor’s of science degree in mechanical engineering from Rensselaer Polytechnic Institute and an MBA in finance from Seton Hall University.
Soluble Film Technology
Since its introduction in the pharmaceutical arena in the early 2000s, Soluble Film (SF) technology has been heralded for its multi-functionality and as the ultimate evolution of the solid oral dosage form. OSFs are a compact, slim, portable, stable, non-fragile, good-tasting way to deliver a unit-dose therapeutic. OSFs are particularly appropriate for certain therapeutic areas and patient types:
- OSF products have found very successful application in systemic drug delivery by sublingual or buccal routes to avoid hepatic first-pass metabolism, GI digestion, and gut-wall metabolism.
- OSFs can offer more rapid absorption and improved overall biovailability.
- For pediatric drugs and adults with dysphagia, rapidly dissolving Oral soluble films solve both the swallowing difficulty of traditional solid dosage forms as well as the ‘spillability’ of oral liquids.
- OSFs are also excellent dosage forms for rescue medications and other drugs involving episodic use, particularly where fast onset of action is a priority.
- OSF are ideal for adults where discreet use, portability and the need to avoid following the dose with water is important.
DepoFilm™ opens new vistas in OSF for new functional shapes, and novel multi-layer compositions, in each case in a single integrated manufacturing process that includes film formation through completion of primary, consumer-ready packaging.
By solving conventionally made-OSF development speed and efficiency problems, DepoFilm™ enables wide market adoption of OSF medications. The DepoFilm™ platform offers compelling value for new chemical entities because it enables rapid formulation prototyping, greatly reduced development times and competitive supply costs. Rapid prototyping allows for buccal, sublingual and intraoral formulations in First Time in Man studies.
For more than a century, film manufacturing has been used in a wide variety of industries. The adoption of industrial film manufacturing for pharmaceutical OSF is a relatively recent development dating back to the early 2000s. NTFP recognized that the path uniformly taken for the manufacture of OSF, using solution-casting of film webs has, prohibited the wider adoption of OSF. NTFP is at the forefront of changing the paradigm by the intuitively simpler, more efficient methodology of film deposition, while at the same time offering a richer offering of film products, all in out integrated manufacturing process.
Traditional solution film-casting of a web are not ideally suited for batch production of pharmaceuticals due to: low process yields resulting from a multi-step process, and film tensile strength and drug uniformity issues.
By solving conventionally made-OSF development speed and efficiency problems, DepoFilm™ enables wide market adoption of OSF medications. The DepoFilm™ platform offers compelling value for new chemical entities because it enables rapid formulation prototyping, greatly reduced development times and competitive supply costs. Rapid prototyping allows for buccal, sublingual and intraoral formulations in First Time in Man studies.
For more than a century, film manufacturing has been used in a wide variety of industries. The adoption of industrial film manufacturing for pharmaceutical OSF is a relatively recent development dating back to the early 2000s. NTFP recognized that the path uniformly taken for the manufacture of OSF, using solution-casting of film webs has, prohibited the wider adoption of OSF. NTFP is at the forefront of changing the paradigm by the intuitively simpler, more efficient methodology of film deposition, while at the same time offering a richer offering of film products, all in out integrated manufacturing process.
Traditional solution film-casting of a web are not ideally suited for batch production of pharmaceuticals due to: low process yields resulting from a multi-step process, and film tensile strength and drug uniformity issues.
DepoFilm™ Technology
Nova Thin Film Pharmaceutical (NTFP)’s patented DepoFilm™ technology is a disruptive advancement in OSF drug delivery with marked improvements in both development timelines and manufacturing efficiency of these versatile dosage forms.
DepoFilm™ Technology
Nova Thin Film Pharmaceutical (NTFP)’s patented DepoFilm™ technology is a disruptive advancement in OSF drug delivery with marked improvements in both development timelines and manufacturing efficiency of these versatile dosage forms. DepoFilm™ opens new vistas in OSF for new functional shapes, and novel multi-layer compositions, in each case in a single integrated manufacturing process that includes film formation through completion of primary, consumer-ready packaging. By solving conventionally made-OSF development speed and efficiency problems, DepoFilm™ enables wide market adoption of OSF medications. The DepoFilm™ platform offers compelling value for new chemical entities because it enables rapid formulation prototyping, greatly reduced development times and competitive supply costs.
Rapid prototyping allows for buccal, sublingual and intraoral formulations in First Time in Man studies. For more than a century, film manufacturing has been used in a wide variety of industries. The adoption of industrial film manufacturing for pharmaceutical OSF is a relatively recent development dating back to the early 2000s. NTFP recognized that the path uniformly taken for the manufacture of OSF, using solution-casting of film webs has, prohibited the wider adoption of OSF. NTFP is at the forefront of changing the paradigm by the intuitively simpler, more efficient methodology of film deposition, while at the same time offering a richer offering of film products, all in out integrated manufacturing process.
Traditional solution film-casting of a web are not ideally suited for batch production of pharmaceuticals due to: low process yields resulting from a multi-step process, and film tensile strength and drug uniformity issues.
Traditional solution film-casting of a web are not ideally suited for batch production of pharmaceuticals due to: low process yields resulting from a multi-step process, and film tensile strength and drug uniformity issues.
DepoFilm™ advantages
The elegance, simplicity and efficiency of DepoFilm™ unlocks OSF product possibilities for our partners and for patients.
1
Higher drug loading compared to conventional soluble film
2
Tolerant of heat-labile API and higher process yields
3
Efficient multi-layer manufacture and novel multi-layer compositions
4
Novel shapes for functionality and branding.
Pipeline
We are working with partners on their proprietary products, as well as products of our own. Our DepoFilm™ technology allows us to focus on targets that have not been previously viable as thin film drug candidates.
Approval
Phase 3
Phase 2
Phase 1
Pre-clinical
Product
(conscious sedation)
NTFP-106
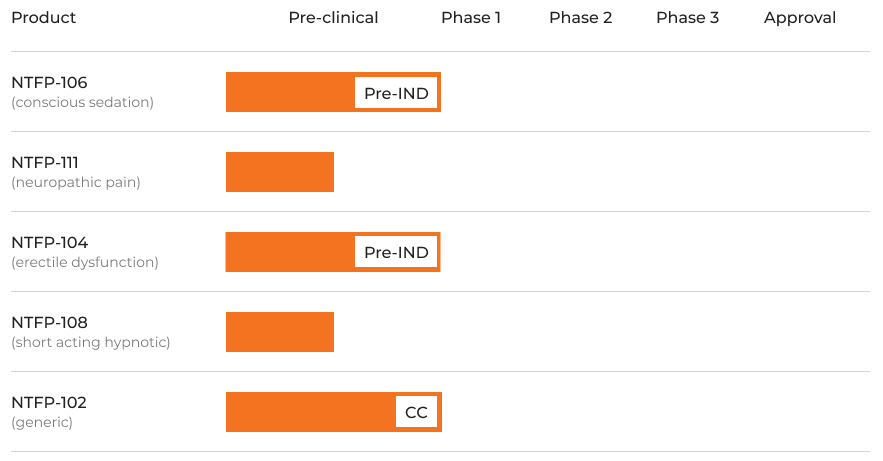
(erectile dysfunction)
NTFP-104
(neuropathic pain)
NTFP-111
(short acting hypnotic)
NTFP-108
(generic)
NTFP-102
Pre-IND
Pre-IND
CC
Be the first to know our news!
Once a month you will hear about our latest features and hottest news. And no spam, of course.
Our news
September 9, 2021
Allowance of sixth DepoFilm™ Patent
August 2, 2021
NOVA Thin Films expands development facility in Greensboro, NC.
July 9, 2021
Allowance of fifth DepoFilm™ Patent
April 6, 2021
Allowance of fourth DepoFilm™ Patent
April 1, 2021
NOVA Thin Films enters into lease for cGMP manufacturing space with Quality Chemical Laboratories LLC.
December 15, 2019
NOVA Thin Films opens development facility in Greensboro, NC.
Show more
November 12, 2019
NOVA Thin Films enters into comprehensive investment and development services agreement with Quality Chemical Laboratories LLC.
October 29, 2019
Formation of NOVA Thin Film Pharmaceuticals LLC under the laws of the State of North Carolina.
March 26, 2019
Issuance of third DepoFilm™ Patent
February 5, 2019
Issuance of second DepoFilm™ Patent
February 27, 2018
Issuance of first DepoFilm™ Patent
April 13, 2017
Filing of first DepoFilm™ Patent
Contact
Feel free to write and call us. We really love to communicate with our clients.
+1-336-808-5359
info@novathinfilm.com
info@novathinfilm.com
Nova Thin Film Pharmaceuticals,
8646 W Market St, Suite 111 Greensboro, NC 27409
8646 W Market St, Suite 111 Greensboro, NC 27409
We use cookies to provide the best site experience.
Ok, don't show again


